Molecular Properties (MoPro)
(Incl. Mollynx)
MoPro is a cristallographic least square refinement package allowing structural or charge density studies of cristal stuctures of variable sizes, ranging from small molecules to biological macromolecules.
The MoProSuite software package
MoProSuite is a software package dedicated to the charge density modeling of crystal structures. It is constituted by two main computational components (MoPro and VMoPro), themselves interfaced by convenient graphical user interfaces (MoProGUI and MoProViewer, respectively).
MoPro is a crystallographic refinement and molecular modeling package. It allows structural and charge density studies of crystal structures of variable sizes, ranging from small molecules to biological macromolecules.It implements the Hansen & Coppens multipolar atom model of electron density. This is necessary to take into account the deformation of electron density arising from the interatomic interactions. These details become visible and quantifiable at subatomic resolution (0.5 Angstrom typically).
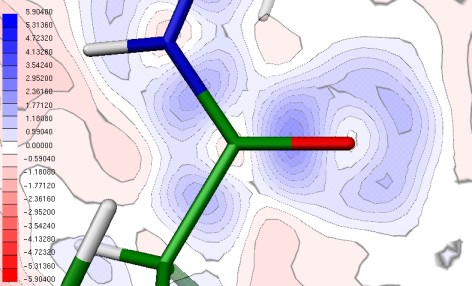
MoPro possesses its own graphical user interface MoProGUI allowing in a user-friendly way to define refinement strategies and to configure a refinement procedure. MoPro is also interfaced with the ELMAM2 experimental multipolar database, which groups the multipolar parameters necessary to describe the charge density of protein and common chemical groups. Hence, the transfer of these parameters allows computing derived electrostatic properties of crystal structures and notably biological macromolecules determined only at usual atomic resolution.
The VMoPro module allows the computation of derived properties based on the multipolar formalism, as molecular electrostatic potential, topological properties or intermolecular interaction energies. It relies on a molecular structure file generated by MoPro and is also interfaced. Its graphical user interface is MoProViewer, a molecular viewer specifically developed for the charge density research which allows, among many features, to graphically configure and execute electron density derived properties computations.
The MoProSuite software has the following most important features
- Multipolar (Hansen & Copppens) and spherical (IAM) models of atomic electron density to refine X-ray structures and charge distribution.
- Lest squares refinement with full matrix inversion or preconditionned conjugate gradients
- Isotropic, anisotropic and anharmonic modeling of atomic thermal motion
- Simple and powerful script language
- Restraints/Constraints on stereochemistry, thermal motion and charge density parameters
- Automated generation of local axes systems
- Automated generation of charge density symmetry and chemical equivalence constraints/restraints
- Automated geometrical constraints/restraints
- Electroneutrality constrain
- For protein crystals, correction of disordered solvent scattering by exponential scaling model or bulk methods
- Analysis tools (stereochemistry, charge density, hydrogen bonding, thermal motion)
- Crystal, molecular or fragments Electrostatic Potential computation
- Total, deformation, valence or multipolar (dipolar, quadripolar ..) electron density computation
- Topological analysis of electron density/ electrostatic potential/Laplacian : critical points, bond ellipticity, Laplacian, energy density Gcp, Vcp.
- Atomic basins and charge integration
- Electric field gradient
- Electrostatic interaction energy computation
- Refinement of Neutron and Electron diffraction crystal structure
- Several file format import/export for molecular structure definition (PDB, Shelxl, CIF…)
- Output Properties displayed in 2D plane, or given sampled on 3D grid (XPLOR or GAUSSIAN CUBE file formats)
- Computation of Hirshfeld surface and contact enrichment ratios
- Modeling of electron density by charged spherical real+virtual atoms
- Estimation of errors on charge density derived properties using the sample standard deviation method.
Reprints on MoPro / MoProViewer
Zarychta, B., Pichon-Pesme, V., Guillot, B., Lecomte, C., & Jelsch, C. (2007). On the application of an experimental multipolar pseudo-atom library for accurate refinement of small-molecule and protein crystal structures. Acta Crystallographica Section A: Foundations of Crystallography, 63(2), 108-125. ![]() (630 Ko)
(630 Ko)
Selection of publications
Bilal, A., Mehmood, A., Noureen, S., Lecomte, C., & Ahmed, M. (2022). CrystEngComm, 24(44), 7758-7770.
Crystal engineering of a co-crystal of antipyrine and 2-chlorobenzoic acid: relative energetic contributions based on multipolar refinement.
Sukumar, N., Kurinov, I., Capel, M. S., Withrow, J., Sukumar, S. L., & Davidson, V. L. (2021).
Ultra-High Resolution and Charge-Density Studies on the Type-I Copper Protein Amicyanin, from Paracoccus Denitrificans. Biophysical Journal, 120(3), 118a-119a.
Mandal, S., Guillot, B., & Munshi, P. (2020). CrystEngComm, 22(26), 4363-4373.
Electron density based analysis of N–H … O= C hydrogen bonds and electrostatic interaction energies
in high-resolution secondary protein structures: insights from quantum crystallographic approaches.
Takaba, K., Tai, Y., Eki, H., Dao, H. A., Hanazono, Y., Hasegawa, K., … & Takeda, K. (2019).
Subatomic resolution X-ray structures of green fluorescent protein. IUCrJ, 6(3), 387-400.
Owczarzak, A., & Kubicki, M. (2018). Crystals, 8(3), 132.
Experimental electron density distribution in two cocrystals of betaines with p-hydroxybenzoic Acid.
Nicolai, B., Fournier, B., Dahaoui, S., Gillet, J. M., & Ghermani, N. E. (2018).
Crystal and electron properties of carbamazepine–aspirin co-crystal. Crystal Growth & Design, 19(2), 1308-1321.
Shukla, R., Claiser, N., Souhassou, M., Lecomte, C., Balkrishna, S. J., Kumar, S., & Chopra, D. (2018). IUCrJ, 5(5), 647-653.
Exploring the simultaneous σ-hole/π-hole bonding characteristics of a Br⋯ π interaction in an ebselen derivative via experimental and theoretical electron-density analysis.
Takaba, K., Takeda, K., Kosugi, M., Tamada, T., & Miki, K. (2017).
Distribution of valence electrons of the flavin cofactor in NADH-cytochrome b5 reductase.
Scientific Reports, 7.
Owczarzak, A., Grześkiewicz, A. M., & Kubicki, M. (2017). Experimental studies of charge density distribution in the crystals of cytisine and N-methylcytisine. Inside the Fake Tobacco. Structural Chemistry, 1-9.
Gianopoulos, C. G., Zhurov, V. V., Minasian, S. G., Batista, E. R., Jelsch, C., & Pinkerton, A. A. (2017). Bonding in Uranium (V) Hexafluoride Based on the Experimental Electron Density Distribution Measured at 20 K. Inorganic Chemistry, 56(4), 1775-1778.
Owczarzak, A., Grześkiewicz, A. M., & Kubicki, M. (2017). Experimental studies of charge density distribution in the crystals of cytisine and N-methylcytisine. Inside the Fake Tobacco. Structural Chemistry, 1-9.
Niranjana Devi, R., Jelsch, C., Israel, S., Aubert, E., Anzline, C., & Hosamani, A. A. (2017). Charge density analysis of metformin chloride, a biguanide anti-hyperglycemic agent. Acta Crystallographica Section B: Structural Science, Crystal Engineering and Materials, 73(1), 10-22.
Wall, M. E. (2016). Quantum crystallographic charge density of urea. IUCrJ, 3(4), 237-246.
Hirano, Y., Takeda, K., & Miki, K. (2016).
Charge-density analysis of an iron–sulfur protein at an ultra-high resolution of 0.48 Å. Nature, 534(7606), 281-284.
Howard, E. I., Guillot, B., Blakeley, M. P., Haertlein, M., Moulin, M., Mitschler, A., … & Petrova, T. (2016). High-resolution neutron and X-ray diffraction room-temperature studies of an H-FABP–oleic acid complex: study of the internal water cluster and ligand binding by a transferred multipolar electron-density distribution. IUCrJ, 3(2), 115-126.
Escudero‐Adán, E. C., Bauzá, A., Frontera, A., & Ballester, P. (2015).
Nature of Noncovalent Carbon‐Bonding Interactions Derived from Experimental Charge‐Density Analysis. ChemPhysChem, 16(12), 2530-2533.
Zarychta, B., Lyubimov, A., Ahmed, M., Munshi, P., Guillot, B., Vrielink, A., & Jelsch, C. (2015). Cholesterol oxidase: ultrahigh-resolution crystal structure and multipolar atom model-based analysis. Acta Crystallographica Section D: Biological Crystallography, 71(4), 954-968.
Kamiński, R., Domagała, S., Jarzembska, K. N., Hoser, A. A., Sanjuan-Szklarz, W. F., Gutmann, M. J., … & Wozńiak, K. (2014). Statistical analysis of multipole-model-derived structural parameters and charge-density properties from high-resolution X-ray diffraction experiments. Acta Crystallographica Section A: Foundations and Advances, 70(1), 72-91.
Lugan, N., Fernández, I., Brousses, R., Valyaev, D. A., Lavigne, G., & Ustynyuk, N. A. (2013). On the incidence of non-covalent intramolecular interligand interactions on the conformation of carbene complexes: a case study. Dalton Transactions, 42(4), 898-901.
Liebschner, D., Jelsch, C., Espinosa, E., Lecomte, C., Chabriere, E., & Guillot, B. (2011).
Topological analysis of hydrogen bonds and weak interactions in protein helices via transferred experimental charge density parameters.
The Journal of Physical Chemistry A, 115(45), 12895-12904.
Fournier, B., Bendeif, E. E., Guillot, B., Podjarny, A., Lecomte, C., & Jelsch, C. (2009). Charge density and electrostatic interactions of fidarestat, an inhibitor of human aldose reductase. Journal of the American Chemical Society, 131(31), 10929-10941.
Liebschner, D., Elias, M., Moniot, S., Fournier, B., Scott, K., Jelsch, C., … & Chabriere, E. (2009). Elucidation of the phosphate binding mode of DING proteins revealed by subangstrom X-ray crystallography. Journal of the American Chemical Society, 131(22), 7879-7886.
Bui, T. T. T., Dahaoui, S., Lecomte, C., Desiraju, G. R., & Espinosa, E. (2009).
The Nature of Halogen⋅⋅⋅ Halogen Interactions: A Model Derived from Experimental Charge‐Density Analysis. Angewandte Chemie International Edition, 48(21), 3838-3841.
Guillot, B., Jelsch, C., Podjarny, A., & Lecomte, C. (2008). Charge-density analysis of a protein structure at subatomic resolution: the human aldose reductase case. Acta Crystallographica Section D: Biological Crystallography, 64(5), 567-588.
Bouhmaida, N., Méndez-Rojas, M. A., Pérez-Benítez, A., Merino, G., Fraisse, B., & Ghermani, N. E. (2010). Experimental electron density study of tetrakis-μ-(acetylsalicylate) dicopper (II): a polymeric structure with Cu··· Cu short contacts. Inorganic chemistry, 49(14), 6443-6452.
Munshi, P., Jelsch, C., Hathwar, V. R., & Guru Row, T. N. (2010).
Experimental and theoretical charge density analysis of polymorphic structures: the case of coumarin 314 dye. Crystal Growth & Design, 10(4), 1516-1526.
Muzet, N., Guillot, B., Jelsch, C., Howard, E., & Lecomte, C. (2003). Electrostatic complementarity in an aldose reductase complex from ultra-high-resolution crystallography and first-principles calculations. Proceedings of the National Academy of Sciences, 100(15), 8742-8747.
Jelsch, C., Teeter, M. M., Lamzin, V., Pichon-Pesme, V., Blessing, R. H., & Lecomte, C. (2000). Accurate protein crystallography at ultra-high resolution: valence electron distribution in crambin. Proceedings of the National Academy of Sciences, 97(7), 3171-3176.





