Summary
Glutathione transferases (GST) are a superfamily of proteins involved in both catalytic and non-catalytic processes. They are primarily known as enzymes of the cellular detoxification system, since they catalyze the addition of glutathione (GSH) to a variety of small compounds to facilitate their removal. GSTs also protect cells against oxidative stress. These catalytic properties explain their involvement in multiple processes in the living world such as drug metabolism, soil remediation or fungicide resistance… GSTs also have various non-catalytic functions: interaction with metabolites for their transport or with proteins. For about ten years we have been carrying out fundamental work on this family of proteins. Our models are GSTs from the plant and fungal kingdoms and recently GSTs from insects and cyanobacteria. In addition to the exploration of the structures of these proteins which led us for example to the discovery of a new Xi class, we study their complexes with ligands. Thus, we have set up original experimental protocols to obtain the crystallographic structures of GSTs in complex with specialized metabolites. These methods allowed, for example, the identification of a flavonoid from cherry tree as a ligand of a GST from the fungus T. versicolor which participates in the mineralization of this species.
Collaborations: Pr. E. Gelhaye (IAM, UMR UL-INRA 1136, Nancy), Dr. C. Cassier-Chauvat (I2BC, UMR CNRS 9198, Paris-Saclay), Dr. F. Neiers (CSGA UMR UB-CNRS-INRA, Dijon).
Glutathione transferases form a superfamilybiomod_fig_1 of proteins involved in both catalytic and non-catalytic processes. GSTs are primarily known as enzymes of the phase II cellular detoxification system, where they predominantly catalyze the nucleophilic addition of glutathione (GSH) to a variety of small non-polar compounds. GSTs also contribute to protect cells against oxidative stress by acting as reductases rather than transferases. These catalytic properties explain their involvement in multiple processes in the living world such as drug metabolism, soil remediation or fungicide resistance… The non-catalytic functions of GST are also very diverse. They interact with specialized metabolites for their transport but also with proteins. For about ten years we have been carrying out fundamental work on this family of proteins. Our models are the GSTs of the plant and fungal kingdoms (collab. IAM, UMR UL-INRA 1136, Nancy) and recently the GSTs of insects and cyanobacteria in collaboration with the CSGA (Dr. F. Neiers, UMR UB-CNRS-INRA, Dijon) and the I2BC (Dr. C. Cassier-Chauvat, UMR CNRS 9198, Paris-Saclay) respectively (i).
Our initial work allowed us to discover a new structural class of GSTs that we called Xi [J. Biol. Chem. 2011, 286, 9162-9173]. This new class is now accepted by the scientific community as it is among the canonical classes of GSTs defined in the UniProt protein database (https://www.uniprot.org/). Xi GSTs are reductases that were initially classified in the Omega class on the basis of sequence alignments, particularly with respect to the yeast isoforms. We have previously solved the crystallographic structure of a yeast isoform and confirmed its membership in class Xi (ii). Furthermore, biochemical and structural studies of several Xi class isoforms in the saprophytic fungus Trametes versicolor have revealed an evolutionary link with the Omega class.
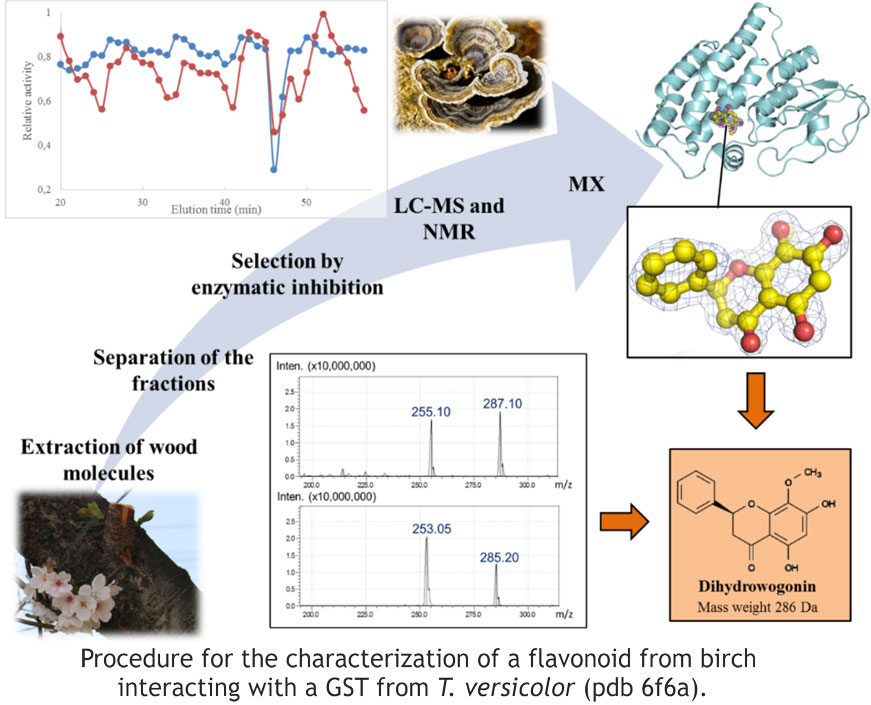
Wood-degrading fungi such as T. versicolor possess complex detoxification systems that allow them to cope with specialized metabolites produced by plants. Although the number of genes encoding GSTs is particularly high in these fungal organisms, little information is available on their target molecules. By combining biochemical, enzymatic and structural approaches, interactions between polyphenols and GSTs of T. versicolor have been demonstrated. To carry out this study, we set up original experimental protocols to obtain the crystallographic structures of complexes between GSTs and polyphenols (iii). In particular, affinity crystallography coupled with spectroscopic data (figure) allowed the identification of a flavonoid from cherry tree as a ligand of T. versicolor GSTs involved in the mineralization of this species (iv). In addition, a systematic review showed agreement between solution interaction data and crystallographic structures of the TvGSTO3S isoform in complex with a variety of hydroxybenzophenones. To quantify this agreement, crystallographic methods developed in the “Modelling” theme of the team, in particular the Charger algorithm, allowed the evaluation of the electrostatic interaction energies of each of the ligands, which are found to be well correlated with the affinities obtained in solution (v). Of course, this approach relies on obtaining crystallographic structures of protein-ligand complexes. Sometimes it remains a difficult step, for instance in the case of complexes between plant GSTs and antocyanins, which form an important class of relatively unstable, water-soluble pigments (vi). Several studies report a role for plant GSTs in their transport into the vacuole. More recently, we have been able to demonstrate an interaction of plant GSTs with several other less water-soluble but more stable classes of flavonoids. A decade ago, GST interactions with flavonoids were suggested; we have now succeeded in characterizing them from a structural point of view. Interestingly, recent studies by our collaborators in Dijon on human GSTs corroborate our results. Thus, plant flavonoids which belong to the human diet and are considered beneficial to health, are probably linked to these GSTs whose exact role remains to be clarified.
References:
- “Functional, Structural and Biochemical Features of Plant Serinyl-Glutathione Transferases”, Sylvestre-Gonon, Law, Schwartz, Robe, Keech, Didierjean, Dubos, Rouhier & Hecker, Front. Plant Sci., 10: 608 (2019). DOI: https://doi.org/10.3389/fpls.2019.00608 HAL: hal-02139339.
- “Crystal structure of Saccharomyces cerevisiae ECM4, a Xi-class glutathione transferase that reacts with glutathionyl-(hydro)quinones”, Schwartz, Didierjean, Hecker, Girardet, Morel-Rouhier, Gelhaye & Favier, PLoS ONE, 11(10): e0164678 (2016). DOI: https://doi.org/10.1371/journal.pone.0164678 HAL: hal-01539425.
- “Fungal Glutathione Transferases as Tools to Explore the Chemical Diversity of Amazonian Wood Extractives”, Perrot, Schwartz, Saiag, Salzet, Dumarçay, Favier, Gerardin, Girardet, Sormani, Morel-Rouhier, Amusant, Didierjean & Gelhaye, ACS Sustain. Chem. Eng., 6(10):13078-13085 (2018). DOI: https://doi.org/10.1021/acssuschemeng.8b02636 HAL: hal-02492224.
- “Molecular recognition of wood polyphenols by phase II detoxification enzymes of the white rot Trametes versicolor”, Schwartz, Perrot, Aubert, Dumarcay, Favier, Gerardin, Morel-Rouhier, Mulliert, Saiag, Didierjean, Gelhaye, Sci. Rep., 8: 8472 (2018). DOI: https://doi.org/10.1038/s41598-018-26601-3 HAL: hal-01843732.
- “A rush to explore protein–ligand electrostatic interaction energy with Charger”, Vuković, Leduc, Jelić-Matošević, Didierjean, Favier, Guillot & Jelsch, Acta Crystallogr D Struct Biol. (2021), 77(10):1292-1304. DOI: https://doi.org/10.1107/s2059798321008433 HAL : hal-03361937.
- “Structural plasticity among glutathione transferase Phi members: natural combination of catalytic residues confers dual biochemical activities”, Pegeot, Mathiot, Perrot, Gense, Hecker, Didierjean & Rouhier, The FEBS Journal, 284: 2442–2463 (2017). DOI: https://doi.org/10.1111/febs.14138 HAL: hal-01593202.